Uttar Pradesh, an Indian state, has mandated that Marion Biotech, the company whose cough syrups were linked to the deaths of 65 children in Uzbekistan, must submit a "corrective and preventive action" report before reopening its factory. The statement was issued by the state's drug controller in response to a Reuters report on an order dated September 14.

This order partially accepted the company's appeal to restart the factory. It allowed Marion to resume production and sales of items that do not contain the ingredient associated with the cough syrup-related deaths but did not specify a timeline for resuming production. The drug controller, Shashi Mohan Gupta, asserted that the Reuters article was misleading and clarified that the Uttar Pradesh government required Marion to submit a Corrective and Preventive Action (CAPA) report to address its deficiencies. Marion Biotech is headquartered in Uttar Pradesh near New Delhi.
The report will be subject to verification by a team of inspectors from federal and state regulatory agencies, as stated by Gupta. He said, "The firm will not carry out any medicine manufacturing work until the final decision of the appellate authority."
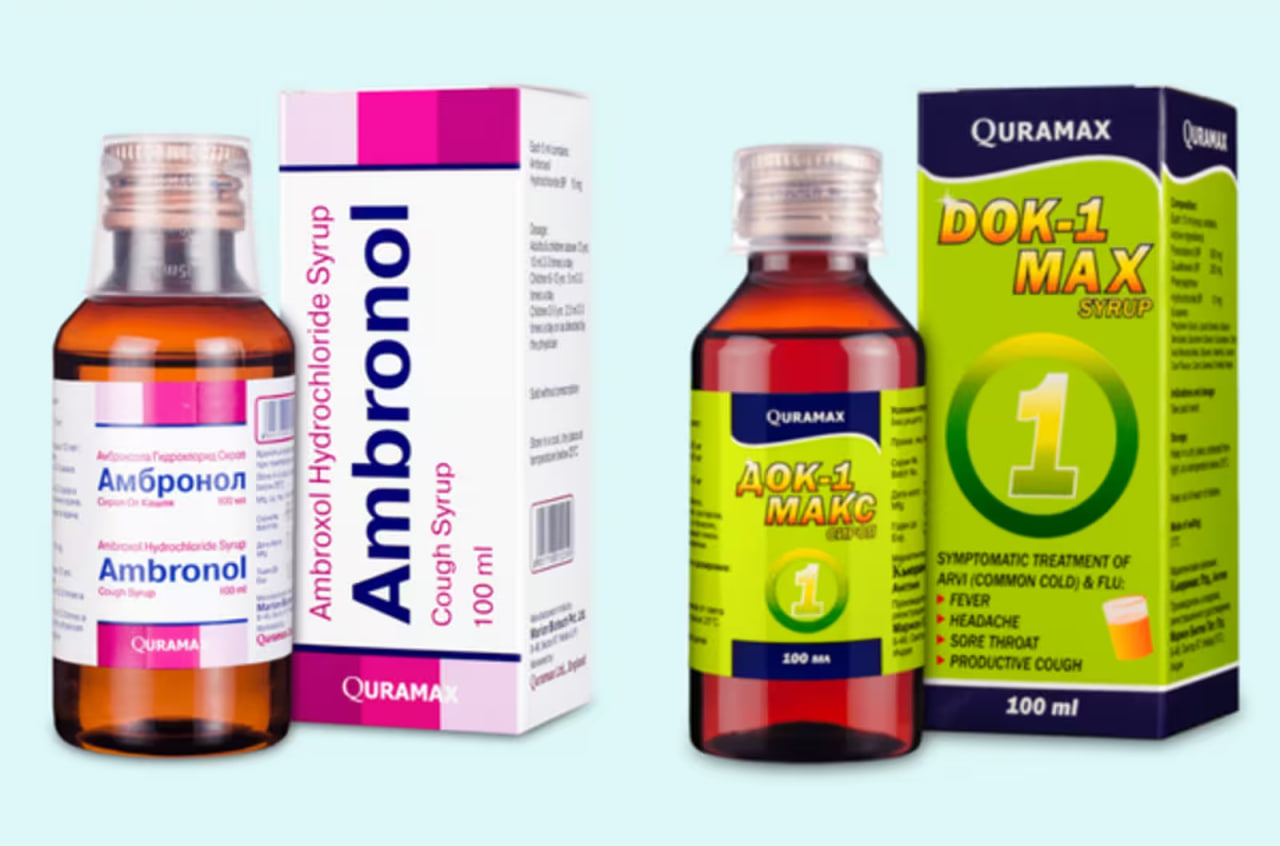
Marion Biotech, among three Indian companies, had their cough syrups associated with the deaths of 141 children in Uzbekistan, Gambia, and Cameroon since the middle of the previous year. The World Health Organization and other agencies linked these incidents, marking one of the most severe cases of mass poisoning worldwide. Gupta, in the September order reported by Reuters, clarified that the Uttar Pradesh government reversed Marion's manufacturing license's cancellation by the state in March on August 11.
The reason cited was the lack of known quality issues in other medications produced by the company. However, Marion was banned from producing products containing propylene glycol, which was found in their Ambronol and DOK-1 Max syrups in unacceptable amounts by Uzbekistan. These chemicals, diethylene glycol (DEG) and ethylene glycol (EG), are not intended for human consumption.

Indian government laboratory tests in January found that 22 samples of Marion-made syrups were "adulterated and spurious," as the country's drug controller informed in March. The pharmaceutical department of India informed parliament the same month that a sample of cough syrup ingredient propylene glycol from Marion's factory contained EG. Marion's former head of operations, Tuhin Bhattacharya, revealed that the company had exported cough syrups for over a decade without testing propylene glycol for impurities like DG or EG.
Follow Daryo's official Instagram and Twitter pages to keep current on world news.
Comments (0)